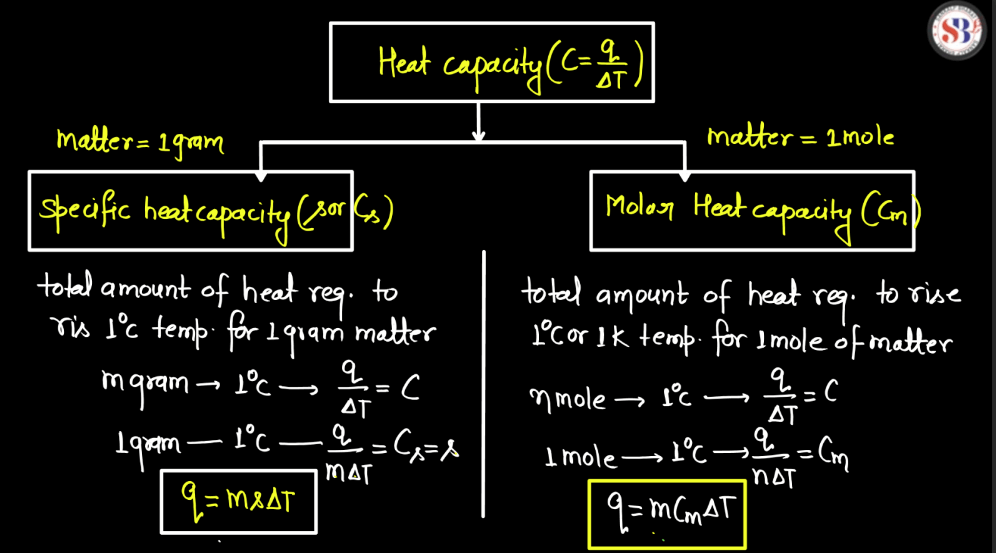
What Is Heat Capacity In Chemistry . In si units, heat capacity (symbol: the heat capacity (c) of a body of matter is the quantity of heat (q) it absorbs or releases when it experiences a. Check out a few specific heat values and example problems. Learn their equations and units. what is specific heat and heat capacity. It is usually expressed as calories. heat capacity is the amount of heat energy required to raise the temperature of a body a specified amount. heat capacity is the amount of heat required to raise the temperature of an object by \(1^\text{o} \text{c}\). heat capacity, ratio of heat absorbed by a material to the temperature change. What is the specific heat of. heat capacity is the amount of heat required to change the temperature of a. The specific heat capacity is the amount of heat it takes to change the temperature of one gram of.
from www.careerpower.in
Learn their equations and units. In si units, heat capacity (symbol: what is specific heat and heat capacity. Check out a few specific heat values and example problems. It is usually expressed as calories. What is the specific heat of. heat capacity is the amount of heat energy required to raise the temperature of a body a specified amount. the heat capacity (c) of a body of matter is the quantity of heat (q) it absorbs or releases when it experiences a. heat capacity is the amount of heat required to raise the temperature of an object by \(1^\text{o} \text{c}\). The specific heat capacity is the amount of heat it takes to change the temperature of one gram of.
What is Heat Capacity Definition, Equations, Examples and Types
What Is Heat Capacity In Chemistry It is usually expressed as calories. Learn their equations and units. What is the specific heat of. heat capacity is the amount of heat required to change the temperature of a. In si units, heat capacity (symbol: heat capacity, ratio of heat absorbed by a material to the temperature change. Check out a few specific heat values and example problems. what is specific heat and heat capacity. heat capacity is the amount of heat energy required to raise the temperature of a body a specified amount. It is usually expressed as calories. heat capacity is the amount of heat required to raise the temperature of an object by \(1^\text{o} \text{c}\). The specific heat capacity is the amount of heat it takes to change the temperature of one gram of. the heat capacity (c) of a body of matter is the quantity of heat (q) it absorbs or releases when it experiences a.
From www.sciencefacts.net
Specific Heat and Heat Capacity Definition, Formula, Values, and Problems What Is Heat Capacity In Chemistry Check out a few specific heat values and example problems. what is specific heat and heat capacity. heat capacity is the amount of heat required to raise the temperature of an object by \(1^\text{o} \text{c}\). The specific heat capacity is the amount of heat it takes to change the temperature of one gram of. Learn their equations and. What Is Heat Capacity In Chemistry.
From www.slideserve.com
PPT Thermochemistry The heat energy of chemical reactions PowerPoint What Is Heat Capacity In Chemistry What is the specific heat of. what is specific heat and heat capacity. heat capacity, ratio of heat absorbed by a material to the temperature change. The specific heat capacity is the amount of heat it takes to change the temperature of one gram of. heat capacity is the amount of heat required to raise the temperature. What Is Heat Capacity In Chemistry.
From www.careerpower.in
What is Heat Capacity Definition, Equations, Examples and Types What Is Heat Capacity In Chemistry Learn their equations and units. What is the specific heat of. The specific heat capacity is the amount of heat it takes to change the temperature of one gram of. the heat capacity (c) of a body of matter is the quantity of heat (q) it absorbs or releases when it experiences a. heat capacity is the amount. What Is Heat Capacity In Chemistry.
From www.thoughtco.com
Heat Capacity Definition in Chemistry What Is Heat Capacity In Chemistry the heat capacity (c) of a body of matter is the quantity of heat (q) it absorbs or releases when it experiences a. What is the specific heat of. Check out a few specific heat values and example problems. heat capacity, ratio of heat absorbed by a material to the temperature change. Learn their equations and units. . What Is Heat Capacity In Chemistry.
From www.youtube.com
Heat Capacity and Specific Heat YouTube What Is Heat Capacity In Chemistry what is specific heat and heat capacity. heat capacity is the amount of heat required to raise the temperature of an object by \(1^\text{o} \text{c}\). Learn their equations and units. In si units, heat capacity (symbol: heat capacity is the amount of heat required to change the temperature of a. the heat capacity (c) of a. What Is Heat Capacity In Chemistry.
From www.slideserve.com
PPT Chapter 16 Reaction Energy PowerPoint Presentation, free download What Is Heat Capacity In Chemistry what is specific heat and heat capacity. Learn their equations and units. In si units, heat capacity (symbol: heat capacity, ratio of heat absorbed by a material to the temperature change. It is usually expressed as calories. Check out a few specific heat values and example problems. heat capacity is the amount of heat required to raise. What Is Heat Capacity In Chemistry.
From askfilo.com
The differences between specific heat capacity and heat capacity are.. What Is Heat Capacity In Chemistry heat capacity is the amount of heat energy required to raise the temperature of a body a specified amount. heat capacity is the amount of heat required to raise the temperature of an object by \(1^\text{o} \text{c}\). the heat capacity (c) of a body of matter is the quantity of heat (q) it absorbs or releases when. What Is Heat Capacity In Chemistry.
From gamesmartz.com
Heat Capacity Definition Easy to Understand What Is Heat Capacity In Chemistry It is usually expressed as calories. Check out a few specific heat values and example problems. In si units, heat capacity (symbol: heat capacity is the amount of heat required to raise the temperature of an object by \(1^\text{o} \text{c}\). the heat capacity (c) of a body of matter is the quantity of heat (q) it absorbs or. What Is Heat Capacity In Chemistry.
From www.careerpower.in
What is Heat Capacity Definition, Equations, Examples and Types What Is Heat Capacity In Chemistry What is the specific heat of. heat capacity is the amount of heat energy required to raise the temperature of a body a specified amount. the heat capacity (c) of a body of matter is the quantity of heat (q) it absorbs or releases when it experiences a. Check out a few specific heat values and example problems.. What Is Heat Capacity In Chemistry.
From www.youtube.com
Specific Heat Capacity Example Problem Physics YouTube What Is Heat Capacity In Chemistry The specific heat capacity is the amount of heat it takes to change the temperature of one gram of. Check out a few specific heat values and example problems. heat capacity is the amount of heat required to raise the temperature of an object by \(1^\text{o} \text{c}\). heat capacity, ratio of heat absorbed by a material to the. What Is Heat Capacity In Chemistry.
From www.downwindkennels.com
Specific Heat Capacity What Is Heat Capacity In Chemistry heat capacity is the amount of heat energy required to raise the temperature of a body a specified amount. What is the specific heat of. In si units, heat capacity (symbol: The specific heat capacity is the amount of heat it takes to change the temperature of one gram of. heat capacity is the amount of heat required. What Is Heat Capacity In Chemistry.
From www.youtube.com
What Is The Difference Between Specific Heat Capacity, Heat Capacity What Is Heat Capacity In Chemistry Learn their equations and units. heat capacity is the amount of heat required to raise the temperature of an object by \(1^\text{o} \text{c}\). Check out a few specific heat values and example problems. It is usually expressed as calories. What is the specific heat of. the heat capacity (c) of a body of matter is the quantity of. What Is Heat Capacity In Chemistry.
From www.youtube.com
General Chemistry Heat Capacity (q=smΔT) [Example 1] YouTube What Is Heat Capacity In Chemistry It is usually expressed as calories. Check out a few specific heat values and example problems. What is the specific heat of. the heat capacity (c) of a body of matter is the quantity of heat (q) it absorbs or releases when it experiences a. In si units, heat capacity (symbol: heat capacity is the amount of heat. What Is Heat Capacity In Chemistry.
From www.youtube.com
Heat Capacity and Specific Heat Capacity Chemistry with Dr. G YouTube What Is Heat Capacity In Chemistry the heat capacity (c) of a body of matter is the quantity of heat (q) it absorbs or releases when it experiences a. heat capacity, ratio of heat absorbed by a material to the temperature change. what is specific heat and heat capacity. What is the specific heat of. heat capacity is the amount of heat. What Is Heat Capacity In Chemistry.
From www.slideserve.com
PPT Chapter 17 Thermochemistry PowerPoint Presentation, free What Is Heat Capacity In Chemistry Learn their equations and units. In si units, heat capacity (symbol: heat capacity is the amount of heat energy required to raise the temperature of a body a specified amount. What is the specific heat of. heat capacity is the amount of heat required to change the temperature of a. what is specific heat and heat capacity.. What Is Heat Capacity In Chemistry.
From www.careerpower.in
What is Heat Capacity Definition, Equations, Examples and Types What Is Heat Capacity In Chemistry Learn their equations and units. It is usually expressed as calories. heat capacity is the amount of heat required to raise the temperature of an object by \(1^\text{o} \text{c}\). What is the specific heat of. heat capacity is the amount of heat required to change the temperature of a. Check out a few specific heat values and example. What Is Heat Capacity In Chemistry.
From www.youtube.com
Heat Capacity and Specific Heat Chemistry Tutorial YouTube What Is Heat Capacity In Chemistry Learn their equations and units. It is usually expressed as calories. what is specific heat and heat capacity. What is the specific heat of. heat capacity, ratio of heat absorbed by a material to the temperature change. heat capacity is the amount of heat required to raise the temperature of an object by \(1^\text{o} \text{c}\). heat. What Is Heat Capacity In Chemistry.
From www.youtube.com
CHEMISTRY 101 Specific heat capacity and calculating heat YouTube What Is Heat Capacity In Chemistry It is usually expressed as calories. heat capacity is the amount of heat required to raise the temperature of an object by \(1^\text{o} \text{c}\). what is specific heat and heat capacity. heat capacity, ratio of heat absorbed by a material to the temperature change. In si units, heat capacity (symbol: heat capacity is the amount of. What Is Heat Capacity In Chemistry.